The majority of things around you (and inside you!) are made of atoms. Atoms are arranged in some sort of order – they can be found in meandering long chain molecules in fats and proteins and the like, in cute little pairs or triplets floating around as gases, or arranged in military order in iron and diamond.
Those materials where the atoms are arranged in repeating patterns, like wallpaper or synchronised swimmers, are referred to as ‘crystalline’ materials. Any material that is not crystalline (or semi-crystalline but that’s a whole other kettle of eels) is considered amorphous.
Crystalline vs Amorphous
When X-rays interact with these crystalline materials, they diffract the radiation a different amount depending on the distance between the atom and its neighbours. When many X-rays interact, these many diffractions build up a pattern that allows us to see how the atoms are arranged in the solid. Since different sized atoms result in different sized gaps between the atoms in the structure, which changes this diffraction pattern.
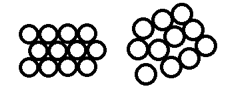
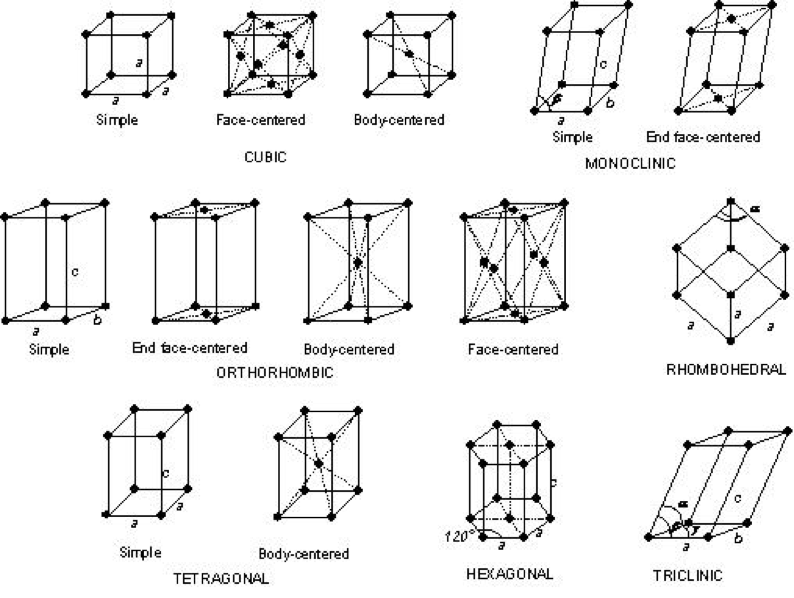
The arrangement, or ‘packing’ of the atoms results in the main pattern of the resulting diffraction. If you think about how you could stack ping pong balls in a plasic tube, or stack apples on a table, that gives you an idea of different crystal structures that might be possible. The basic crystal structures are shown below.
The way these atoms are arranged, the crystal structure, determines how a crystal is formed and grows over time – you may have noticed the little hematite cubes you can see at the markets, or the way some gemstones form a hexagonal shape. This arrangement of atoms determined how the crystal will look on a macro scale. Sometimes individual atoms in a crystal structure will be substituted with another atom type (for example manganese for iron), which usually won’t change the overall structure, but might change the colour of the crystal or the way the light bounces through it.
X-ray diffraction analysis uses this unique interaction between X-ray radiation and crystalline materials to build a diffraction pattern which is then compared to a huge database of reference patterns in order to determine which crystal structures are present in the material. This allows up to tell the difference between Halite and Sylvite, and between Goethite and Hematite without any trouble.
Want to identify your crystals? Ask for X-ray Diffraction!
Nimue Pendragon